Purpose Of A Distillation Unit . in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation involves boiling the solution and. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. distillation is the most widely used technique to separate liquid mixtures. However, it uses large amounts of energy. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation separates a soluble substance from its solvent based on differences in boiling points.
from www.wermac.org
distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. Distillation involves boiling the solution and. However, it uses large amounts of energy. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation is the most widely used technique to separate liquid mixtures. distillation separates a soluble substance from its solvent based on differences in boiling points. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere.
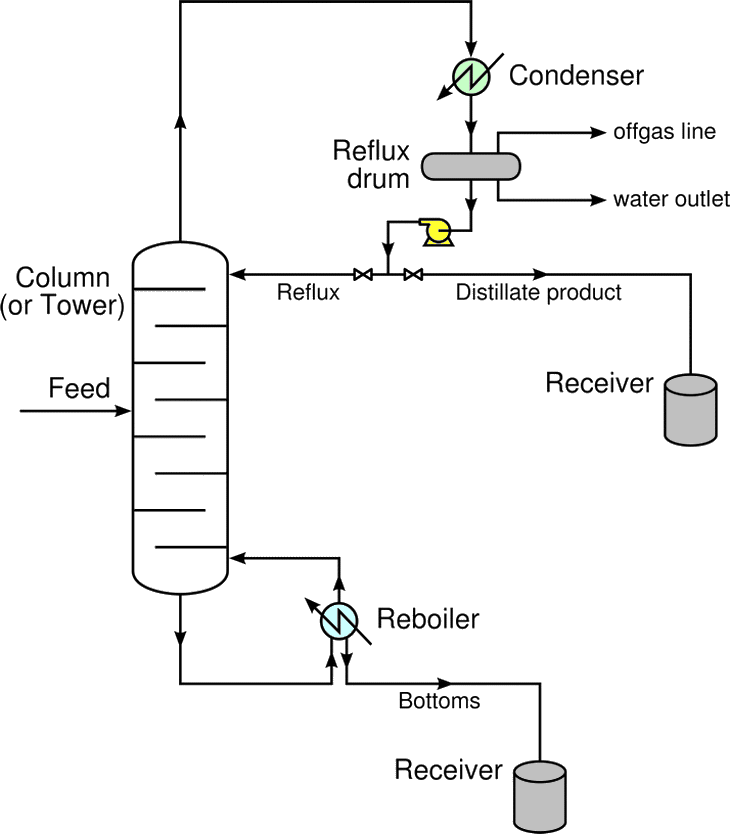
Distillation Column Basic Distillation Equipment and Operation
Purpose Of A Distillation Unit distillation separates a soluble substance from its solvent based on differences in boiling points. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. However, it uses large amounts of energy. distillation separates a soluble substance from its solvent based on differences in boiling points. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation involves boiling the solution and. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is the most widely used technique to separate liquid mixtures.
From chemicalengineeringworld.com
Types of Distillation Chemical Engineering World Purpose Of A Distillation Unit distillation is the most widely used technique to separate liquid mixtures. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation involves boiling the solution and. distillation. Purpose Of A Distillation Unit.
From dxozgknhl.blob.core.windows.net
The Purpose Of Vacuum Distillation at Ronnie Allen blog Purpose Of A Distillation Unit distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation separates a soluble substance from its solvent based on differences in boiling points. Distillation involves boiling the solution and. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is. Purpose Of A Distillation Unit.
From speichim.com
Main principes of distillation Speichim Processing Valls Química Purpose Of A Distillation Unit distillation is the most widely used technique to separate liquid mixtures. distillation separates a soluble substance from its solvent based on differences in boiling points. Distillation involves boiling the solution and. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. However, it uses large amounts of energy.. Purpose Of A Distillation Unit.
From studylib.net
Simple Distillation Purpose Of A Distillation Unit distillation separates a soluble substance from its solvent based on differences in boiling points. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is the most widely used technique to separate liquid mixtures. However, it uses large amounts of energy. distillation is a separation technique. Purpose Of A Distillation Unit.
From www.wermac.org
Distillation Column Basic Distillation Equipment and Operation Purpose Of A Distillation Unit distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation separates a soluble substance from its solvent based on differences in boiling points. distillation is the most widely used technique to separate liquid mixtures. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting. Purpose Of A Distillation Unit.
From www.shutterstock.com
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous Purpose Of A Distillation Unit distillation separates a soluble substance from its solvent based on differences in boiling points. distillation is the most widely used technique to separate liquid mixtures. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. distillation refers to the selective boiling and subsequent condensation of a component in. Purpose Of A Distillation Unit.
From www.etsy.com
Distillation Apparatus Diagram Poster Printable Lab Tools Etsy Purpose Of A Distillation Unit Distillation involves boiling the solution and. distillation is the most widely used technique to separate liquid mixtures. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation separates a soluble substance. Purpose Of A Distillation Unit.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Purpose Of A Distillation Unit in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation separates a soluble substance from its solvent based on differences in boiling points. Distillation involves boiling the solution and. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid. Purpose Of A Distillation Unit.
From exoqonhdf.blob.core.windows.net
Purpose Of Distillation Plant at Joshua Kelley blog Purpose Of A Distillation Unit distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. Distillation involves boiling the solution. Purpose Of A Distillation Unit.
From www.gustawater.com
The Ultimate Guide to Distillation and Distillation Columns Purpose Of A Distillation Unit distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation separates a soluble substance from its solvent based on differences in boiling points. distillation is the most. Purpose Of A Distillation Unit.
From www.newworldencyclopedia.org
Fractional distillation New World Encyclopedia Purpose Of A Distillation Unit However, it uses large amounts of energy. distillation is the most widely used technique to separate liquid mixtures. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. Distillation involves boiling the solution. Purpose Of A Distillation Unit.
From www.indiamart.com
Horizontal Water Distillation Unit , Single, Lab Tech Scientific Works Purpose Of A Distillation Unit distillation is the most widely used technique to separate liquid mixtures. However, it uses large amounts of energy. distillation separates a soluble substance from its solvent based on differences in boiling points. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. distillation is a separation technique used. Purpose Of A Distillation Unit.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. Purpose Of A Distillation Unit distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. However, it uses large amounts of energy. distillation is used to separate liquids from nonvolatile solids, as in the. Purpose Of A Distillation Unit.
From www.wermac.org
Distillation Column Basic Distillation Equipment and Operation Purpose Of A Distillation Unit distillation is the most widely used technique to separate liquid mixtures. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is a separation technique used to separate. Purpose Of A Distillation Unit.
From www.youtube.com
Steam Distillation Technique Basic Principles and Techniques in Purpose Of A Distillation Unit distillation is the most widely used technique to separate liquid mixtures. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation refers to the selective boiling and subsequent condensation of a component in a liquid. distillation is used to separate liquids from nonvolatile solids, as in. Purpose Of A Distillation Unit.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Purpose Of A Distillation Unit distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation separates a soluble substance from its solvent based on differences in boiling points. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation refers to the. Purpose Of A Distillation Unit.
From www.researchgate.net
Process flow diagram of Vacuum Distillation Unit Download Scientific Purpose Of A Distillation Unit Distillation involves boiling the solution and. distillation separates a soluble substance from its solvent based on differences in boiling points. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented. However, it uses large amounts of energy. distillation refers to the selective boiling and subsequent condensation of a component. Purpose Of A Distillation Unit.
From chemistnotes.com
Distillation Definition and Types of Distillation Chemistry Notes Purpose Of A Distillation Unit distillation is the most widely used technique to separate liquid mixtures. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation involves boiling the solution and. distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. distillation. Purpose Of A Distillation Unit.